Questions | Answer | Explanations |
31 What is the net charge on an ion that has 9 protons, 11 neutrons, and 10 electrons?
(1) 1+ (3) 1–
(2) 2+ (4) 2– | 3 | more electrons means it is negative |
32 Which two particles make up most of the mass of a hydrogen-2 atom?
(1) electron and neutron
(2) electron and proton
(3) proton and neutron
(4) proton and positron | 3 | H-2 has a proton and a neutron |
33 Which statement explains why sulfur is classified as a Group 16 element?
(1) A sulfur atom has 6 valence electrons.
(2) A sulfur atom has 16 neutrons.
(3) Sulfur is a yellow solid at STP.
(4) Sulfur reacts with most metals. | 1 | group 16, 6 valence electrons |
34 How do the atomic radius and metallic properties of sodium compare to the atomic radius and metallic properties of phosphorus?
(1) Sodium has a larger atomic radius and is more metallic.
(2) Sodium has a larger atomic radius and is less metallic.
(3) Sodium has a smaller atomic radius and is more metallic.
(4) Sodium has a smaller atomic radius and is less metallic. | 1 | table S for radius more metallic bottom left of the PT |
35 A compound has a molar mass of 90. grams per mole and the empirical formula CH2O. What is the molecular formula of this compound?
(1) CH2O (3) C3H6O3
(2) C2H4O2 (4) C4H8O4 | 3 | CH2O=30g/mol 90/30; so 3 times this |
36 At standard pressure, a certain compound has a low boiling point and is insoluble in water. At STP, this compound most likely exists as
(1) ionic crystals
(2) metallic crystals
(3) nonpolar molecules
(4) polar molecules | 3 | water is polar and like dissolves like, so nonpolar low BP covalent |
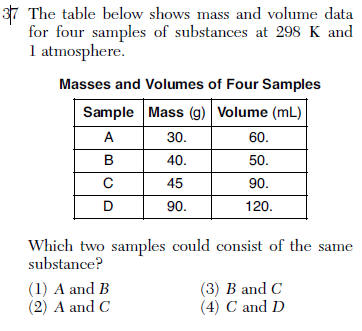 | 2 | compare densities mass/volume |
38 Which group on the Periodic Table of the Elements contains elements that react with oxygen to form compounds with the general formula X2O?
(1) Group 1 (3) Group 14
(2) Group 2 (4) Group 18 | 1 | X has a charge of +1 so group 1 |
39 An unsaturated solution is formed when 80. grams of a salt is dissolved in 100. grams of water at 40.°C. This salt could be
(1) KCl (3) NaCl
(2) KNO3 (4) NaNO3 | 4 | Below the line on TABLE G at 40C |
40 Which kelvin temperature is equal to 56°C?
(1) –329 K (3) 217 K
(2) –217 K (4) 329 K | 4 | add 273 |
