Questions
(1) The concentration of the products is greater than the concentration of the reactants.
(2) The concentration of the products is less than the concentration of the reactants.
(3) The concentration of the products and the concentration of the reactants are equal.
(4) The concentration of the products and the concentration of the reactants are constant.
concentrations constant Rates are equal
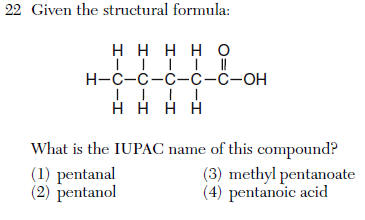
acid -anoic acid
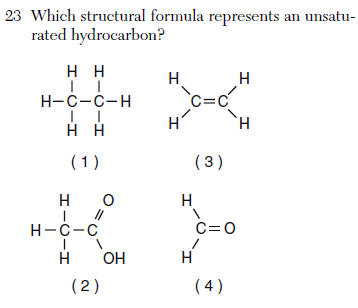
unsaturated (contains a double or triple bond)
(1) alpha particle, beta particle, gamma radiation
(2) gamma radiation, alpha particle, beta particle
(3) positron, alpha particle, neutron
(4) neutron, positron, alpha particle
positron +1
alpha +2
molecules that contain two carbon atoms, one oxygen atom, and six hydrogen atoms. These two substances must be
(1) isomers of each other
(2) isotopes of each other
(3) the same compound
(4) the same hydrocarbon
CH3CH2CH3 + Br2==> CH3CH2CH2Br + HBr
This organic reaction is best classified as
(1) an addition reaction
(2) an esterification reaction
(3) a polymerization reaction
(4) a substitution reaction
(1) higher energy and higher entropy
(2) higher energy and lower entropy
(3) lower energy and higher entropy
(4) lower energy and lower entropy
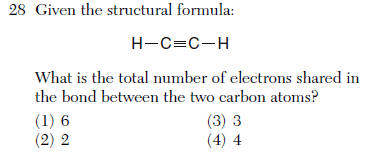
(1) CH4 (3) KH
(2) CaH2 (4) NH3
(1) NO3– (3) OH–
(2) Cl– (4) H–