Questions | Answer | Explanations |
1 What is the electron configuration of a sulfur atom in the ground state?
| 4 | Ground State configurations are listed on the Periodic Table for each element. |
2 The modern model of the atom shows that electrons are
| 2 | Electrons are found in orbitals outside the nucleus. Protons and neutrons are located in the nnucleus. |
3 Which compound contains ionic bonds?
| 3 | Ionic compounds can be easily identified because they are on opposite ends of the periodic table. One metals and one nonmetal. **Note Incorrect answers are all covalent (2 nonmetals) |
4 All the isotopes of a given atom have
| 3 | Definition-Isotope KNOW IT |
5 What are two properties of most nonmetals?
| 1 | Use Table S Pick a nonmetal (fluorine) and compare it to a Metal (sodium) |
6 Which element is classified as a noble gas at STP?
| 3 | noble gas-Group 18 |
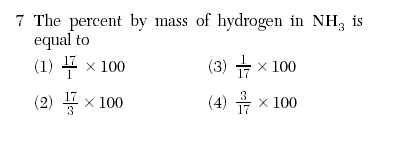 | 4 | Nitrogen=14 hydrogen= 3 *1=3 NH3=17 (part/whole) x100 |
8 Metallic bonding occurs between atoms of
| 2 | Metals, so pick a metal |
| 9 Atoms of the same element that have different numbers of neutrons are classified as
| 4 | Definition of Isotope KNOW IT |
10 Compared to the radius of a chlorine atom, the radius of a chloride ion is
| 2 | Metals lose electrons and get smaller Nonmetals gain and get bigger |