Questions | Answer | Explanations |
1 The modern model of the atom is based on the work of (1) one scientist over a short period of time
(2) one scientist over a long period of time
(3) many scientists over a short period of time
(4) many scientists over a long period of time
| 4 | Dalton,Thompson,Rutherford,Bohr... |
2 Which statement is true about the charges assigned to an electron and a proton? (1) Both an electron and a proton are positive.
(2) An electron is positive and a proton is negative.
(3) An electron is negative and a proton is positive.
(4) Both an electron and a proton are negative.
| 3 | protons + electrons - neutrons neutral |
3 In the wave-mechanical model, an orbital is a region of space in
an atom where there is (1) a high probability of finding an electron
(2) a high probability of finding a neutron
(3) a circular path in which electrons are found
(4) a circular path in which neutrons are found
| 1 | definition- electrons travel in waves and we we have an idea where the electron is found using probability |
4 What is the charge of the nucleus in an atom of oxygen-17? (1) 0 (3) +8
(2) –2 (4) +17
| 3 | nuclear charge = # protons= +8 |
5 Which pair of symbols represents a metalloid and a noble gas? (1) Si and Bi (3) Ge and Te
(2) As and Ar (4) Ne and Xe
| 2 | metalloids found on staircase and Noble Gases are group 18 |
6 Which statement describes a chemical property of iron? (1) Iron can be flattened into sheets.
(2) Iron conducts electricity and heat.
(3) Iron combines with oxygen to form rust.
(4) Iron can be drawn into a wire.
| 3 | chemical property refers to a reaction |
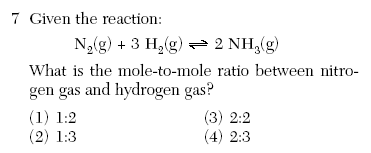 | 2 | use the coefficients which is 1 to 3 |
8 What is the percent by mass of oxygen in propanal, CH3CH2CHO? (1) 10.0% (3) 38.1%
(2) 27.6% (4) 62.1%
| 2 | gram formula mass= 3(12)+6(1)+16=58 (16/58) * 100%= 27.6% |
9 Covalent bonds are formed when electrons are (1) transferred from one atom to another
(2) captured by the nucleus
(3) mobile within a metal
(4) shared between two atoms
| 4 | Covalent shared electrons Ionic Transferred |
10 Which type of molecule is CF4? (1) polar, with a symmetrical distribution of charge
(2) polar, with an asymmetrical distribution of charge
(3) nonpolar, with a symmetrical distribution of charge
(4) nonpolar, with an asymmetrical distribution of charge
| 3 | SNAP CF4 is Symmetrical is Nonpolar Asymmetrical is Polar |