Questions
(1) mass number and atomic number of an atom
(2) organization of atoms in a crystal
(3) radioactive nature of some atoms
(4) spectra of elements with multielectron atoms
electrons==> spectra
(1) electron shells (3) neutrons
(2) valence electrons (4) protons
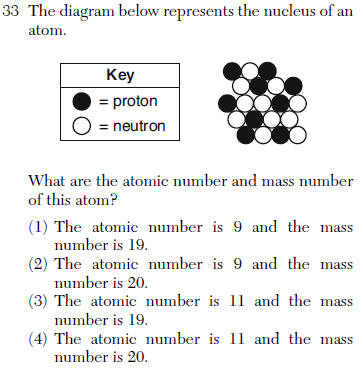
mass number= protons + neutrons
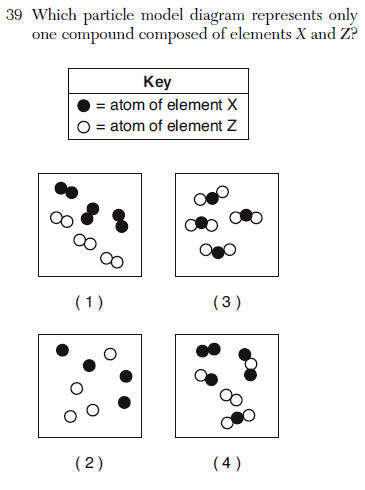
(1) one chlorine atom
(2) two chlorine atoms
(3) one sodium atom
(4) two sodium atoms
(1) 1% (3) 3%
(2) 2% (4) 4%
use Table S for actual density 2.698
(2.81*2.698/2.698) * 100%
2H2 + O2 ==>2H2O
What is the total mass of water formed when 8 grams of hydrogen reacts completely with 64 grams of oxygen?
(1) 18 g (3) 56 g
(2) 36 g (4) 72 g
if 8g reacts completely with 64g you get 72 grams produced
(1) ammonia
(2) methane
(3) sodium nitrate
(4) potassium chloride
NaNO3
3 different elements and 1 is a metal
(1) 65 K (3) 65°C
(2) 45 K (4) 45°C
convert to K by adding 273 to C
Cu + S==> CuS + energy
Which statement explains why the energy term is written to the right of the arrow?
(1) The compound CuS is composed of two metals.
(2) The compound CuS is composed of two nonmetals.
(3) Energy is absorbed as the bonds in CuS form.
(4) Energy is released as the bonds in CuS form.