Questions
(1) cooling curve
(2) heating curve
(3) ionization energy diagram
(4) potential energy diagram
(1) 200. K and 50.0 kPa
(2) 200. K and 200.0 kPa
(3) 600. K and 50.0 kPa
(4) 600. K and 200.0 kPa
low pressure and high temperatures
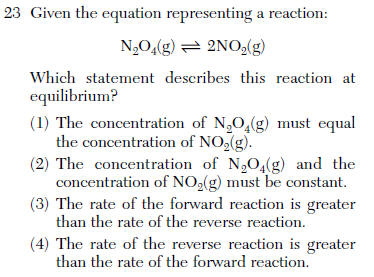
Concentrations constant and the rates are equal
(1) propanal (3) propene
(2) propane (4) propyne
(1) molecular formulas
(2) structural formulas
(3) total number of atoms per molecule
(4) total number of bonds per molecule
(1) base + acid Å=> salt + water
(2) base +salt => water + acid
(3) salt + acid =>base + water
(4) salt + water=> acid + base
(1) aldehyde (3) Arrhenius acid
(2) alcohol (4) Arrhenius base
(1) H+ donor (3) OH- donor
(2) H+ acceptor (4) OH- acceptor
H+ acceptor is and base
(1) an alpha particle (3) a neutron
(2) a beta particle (4) a positron
positron same mass as beta
(1) a ground-state electron
(2) a stable nucleus
(3) an excited electron
(4) an unstable nucleus