Before atomic numbers were known, Mendeleev developed a classification system for the 63 elements known in 1872, using oxide formulas and atomic masses. He used an R in the oxide formulas to represent any element in each group. The atomic mass was listed in parentheses after the symbol of each element. A modified version of Mendeleev's classification system is shown in the table below.
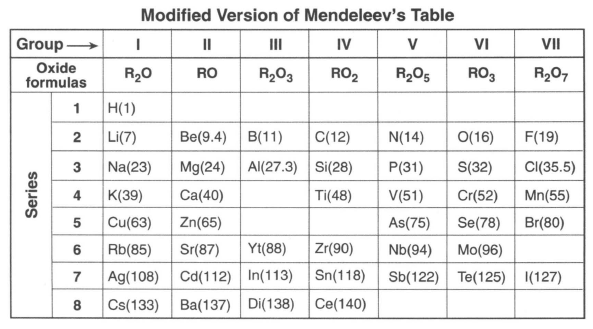
66 Identify one characteristic used by Mendeleev to develop his classification system of the elements. [1]
Highlight box to reveal answer
increasing atomic mass atomic mass oxide formulas (it was in the first sentence) |
67 Based on Mendeleev's oxide formula, what is the number of electrons lost by each atom of the elements in Group III? [1]
Highlight box to reveal answer
68 Based on Table