Questions
Explanations
36 Given the balanced equation representing a reaction:
2NO + O2 --> 2NO + energy
The mole ratio of NO to NO is
(1) 1 to 1 (3) 3 to 2
(2) 2 to 1 (4) 5 to 2

Which type of bonding is present when valence electrons move within the sample?
(1) metallic bonding (2) hydrogen bonding
(3) covalent bonding (4) ionic bonding"positive ion immersed in a sea of electrons"
38 Given the formula representing a molecule:
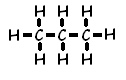
Which statement explains why the molecule is nonpolar?
(l) Electrons are shared between the carbon atoms and the hydrogen atoms.
(2) Electrons are transferred from the carbon atoms to the hydrogen atoms.
(3) The distribution of charge in the molecule is symmetrical.
(4) The distribution of charge in the molecule is asymmetrical.
symmetrical nonpolar asymmetrical polar
39 A solid sample of a compound and a liquid sample of the same compound are each tested for electrical conductivity. Which test conclusion indicates that the compound is ionic?
(1) Both the solid and the liquid are good conductors.
(2) Both the solid and the liquid are poor conductors.
(3) The solid is a good conductor, and the liquid is a poor conductor.
(4) The solid is a poor conductor, and the liquid is a good conductor.
solids can't move, liquids can
40 Which statement explains why 10.0 mL of a 0.50 M H2SO4(aq) solution exactly neutralizes 5.0 mL of a 2.0 M NaOH(aq) solution?
(1) The moles of H+(aq) equal the moles of OH-(aq).
(2) The moles of H2SO4(aq) equal the moles of NaOH(aq).
(3) The moles of H2SO(aq) are greater than the moles of NaOH(aq).
(4) The moles of H+(aq) are greater than the moles of OH-(aq).