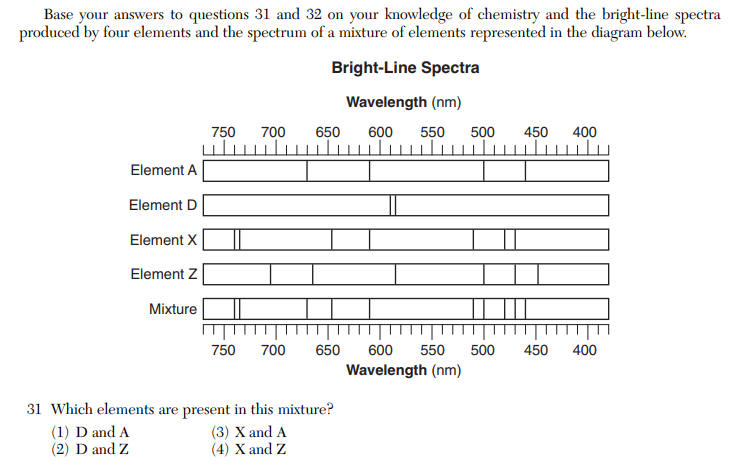
Questions
Explanations
(1) absorbed as an atom loses an electron
(2) absorbed as an atom gains an electron
(3) released as an electron moves from a lower energy state to a higher energy state
(4) released as an electron moves from a higher energy state to a lower energy state

(1) 26 u (3) 52 u
(2) 45 u (4) 71 u
(1) 2-7-6 (3) 2-8-8-1
(2) 2-8-5 (4) 2-8-7-2