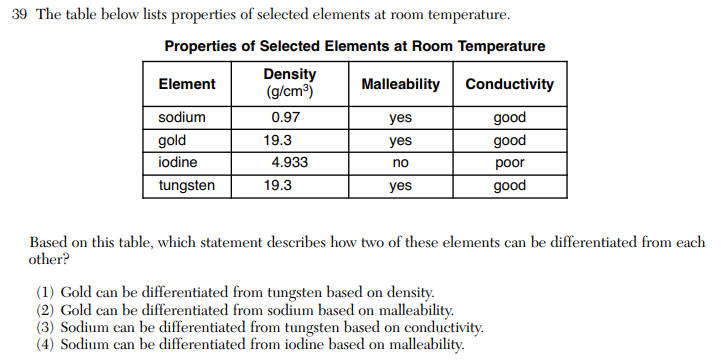
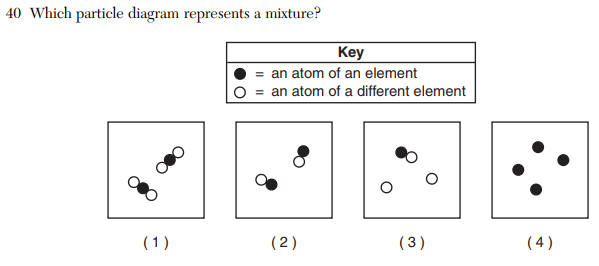
Questions
Explanations
(1) 19 (3) 23
(2) 20 (4) 42
(1) 1.0 mol (3) 3.0 mol
(2) 2.0 mol (4) 4.0 mol
Mg(s) + 2HCl(aq) → MgCl2(aq) + H2(g)
Which type of chemical reaction is represented by the equation?
(1) synthesis
(2) decomposition
(3) single replacement
(4) double replacement