|
Questions | Answer | Links | Explanations |
| 41 An atom of which element reacts with an atom of hydrogen to form a bond with the greatest degree of polarity? (1) carbon (3) nitrogen (2) fluorine (4) oxygen | | | |
| 42 What is the concentration of an aqueous solution that contains 1.5 moles of NaCl in 500. milliliters of this solution? (1) 0.30 M (3) 3.0 M (2) 0.75 M (4) 7.5 M | | | |
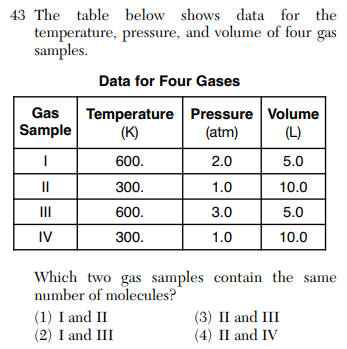 | | | |
|
| 44 Based on Table I, what is the �H value for the production of 1.00 mole of NO2(g) from its elements at 101.3 kPa and 298 K? (1) +33.2 kJ (3) +132.8 kJ (2) -33.2 kJ (4) -132.8 kJ | | | |
| 45 Which equation represents an addition reaction? (1) C3H8 + Cl2 → C3H7Cl + HCl (2) C3H6 + Cl2 → C3H6Cl2 (3) CaCl2 + Na2CO3 → CaCO3 + 2NaCl (4) CaCO3 → CaO + CO2 | | | |
|
