Question 66-69
| ||||||
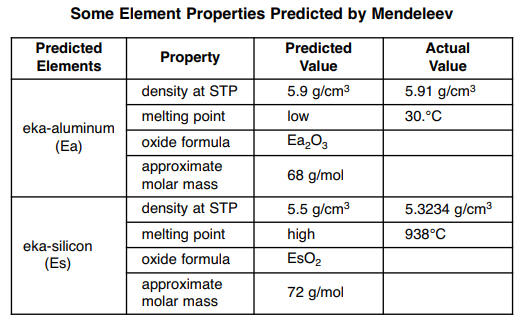
Base your answers to questions 66 through 69 on the information below and on your knowledge of chemistry. In the late 1800s, Dmitri Mendeleev developed a periodic table of the elements known at that time. Based on the pattern in his periodic table, he was able to predict properties of some elements that had not yet been discovered. Information about two of these elements is shown in the table below.
66 Identify the phase of Ea at 310. K. [1]
67 Write a chemical formula for the compound formed between Ea and Cl. [1]
68 Identify the element that Mendeleev called eka-silicon, Es. [1]
69 Show a numerical setup for calculating the percent error of Mendeleev’s predicted density of Es. [1]
on to Questions 70-73 | ||||||