|
|
|
Questions
|
Answer |
Links |
Explanations
|
| 36 Which formula represents calcium hydride?
1. CaH
2. CaH2
3. CaOH
4. Ca(OH)2 |
2 |
|
trickier question. not hydroxide so 3 and 4 are out
Ca is 2+ and Hydride H is 1-
so CaH2 |
| 37 What is the number of moles in a 78.8-gram sample
of MgCO3 (gram-formula mass = 84.3 g/mol)?
1. 0.949 mol
2. 0.935 mol
3. 0.843 mol
4. 1.070 mol |
2 |
|
equations are on Table T
78.8g /84.3g/mol= 0.935 mol |
| 38 Given the equation representing a reaction: F2(g)
+ 2 KCl(aq) --> 2 KF(aq) + Cl2(g)
Which type of chemical reaction is represented by the equation?
1. synthesis
2. decomposition
3. single replacement
4. double replacement |
3 |
|
element + compound --> compound + element |
|
|
| 39 based on Table H, which compound has the strongest
intermolecular forces at 60 kPa? 1.
ethanoic acid
2. ethanol
3. propanone
4. water |
1 |
|
strongest forces will require the highest temperature
to create 60kPa of pressure |
| 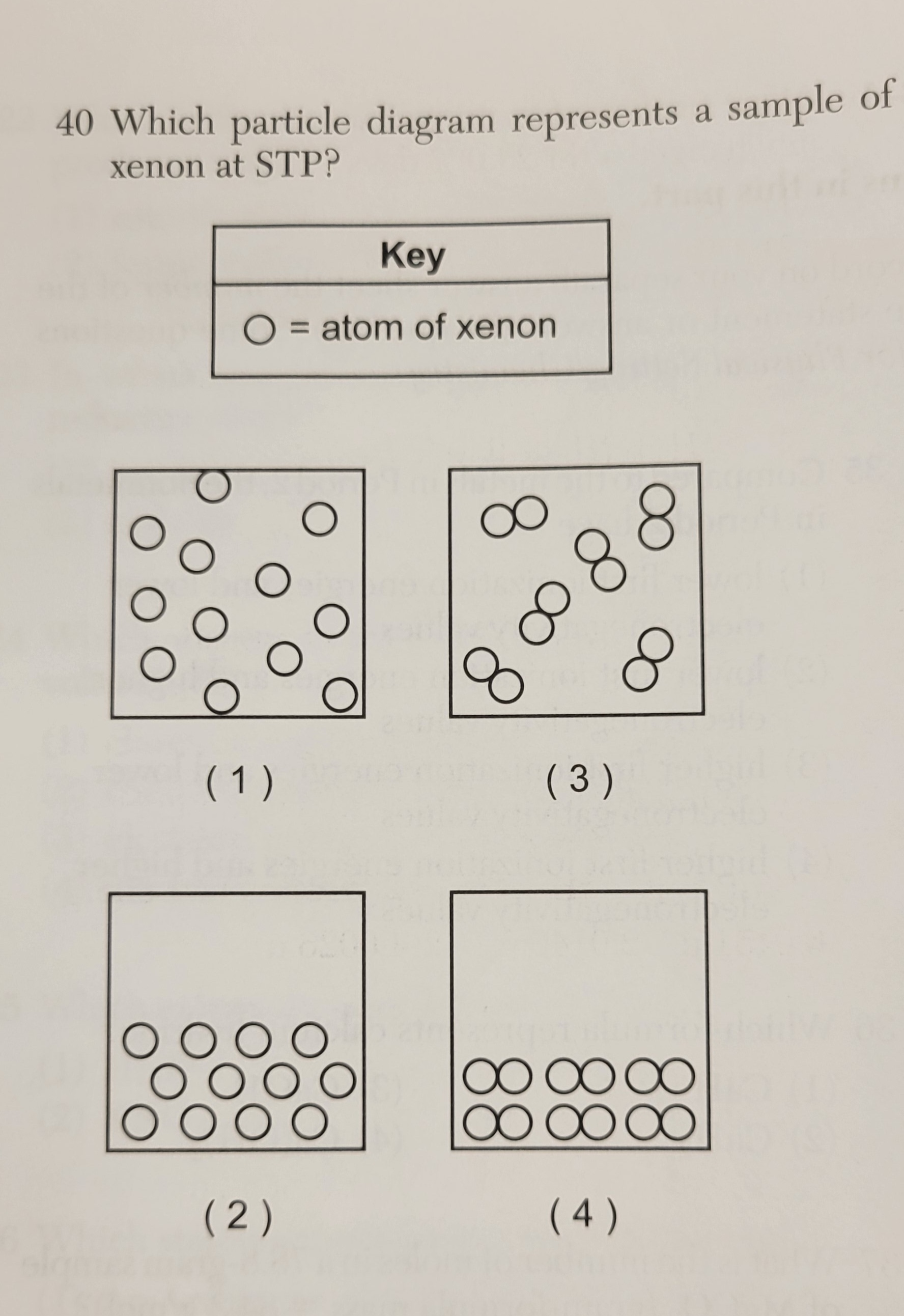 |
1 |
|
Xenon is a Noble gas even at STP it is a
monoatomic gas. Pick to picture with it separated and not
paired up. 1. is a monoatomic gas
3. is a diatomic gas
2,4 not gases
|
|
|
