Questions
Explanations
1. 0.10 M NaCl(aq)
2. 0.010 M NaCl(aq)
3. 0.10 M C6H12O6(aq)
4. 0.010 M C6H12O6(aq)
1 has the large concentration and is the better conductor
1. 1/8
2. 1/2
3. 1/3
4. 1/4
24.063 d/ 8.021d= 3 half lives
1--> 1/2-->1/4--> 1/8
|
2 |
H + |
2 |
H --> |
3 |
He + |
1 |
n + Energy |
|
1 |
1 |
2 |
0 |
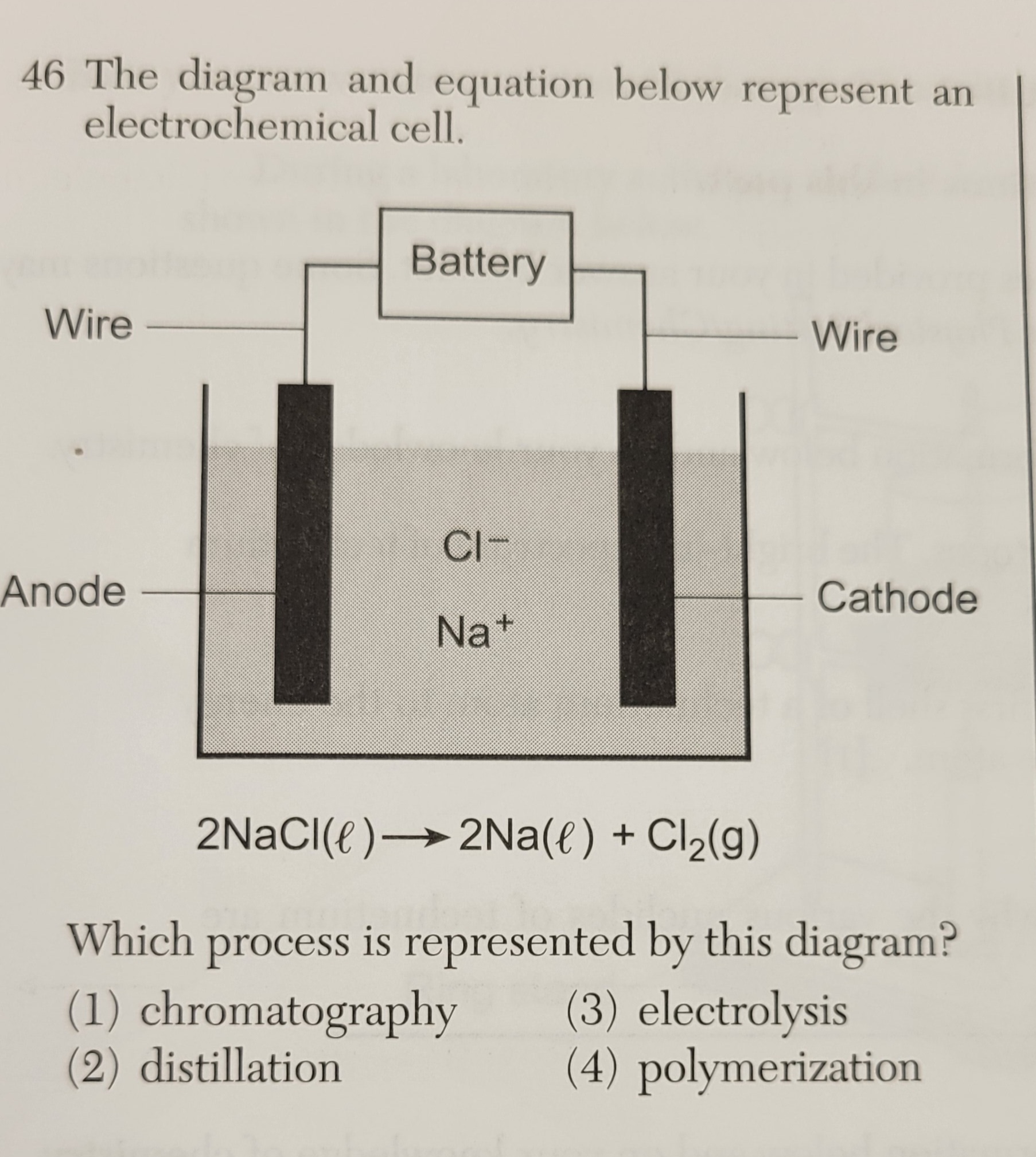
Which type of reaction is represented by the equation?
1. nuclear fission
2. nuclear fusion
3. combination
4. substitution
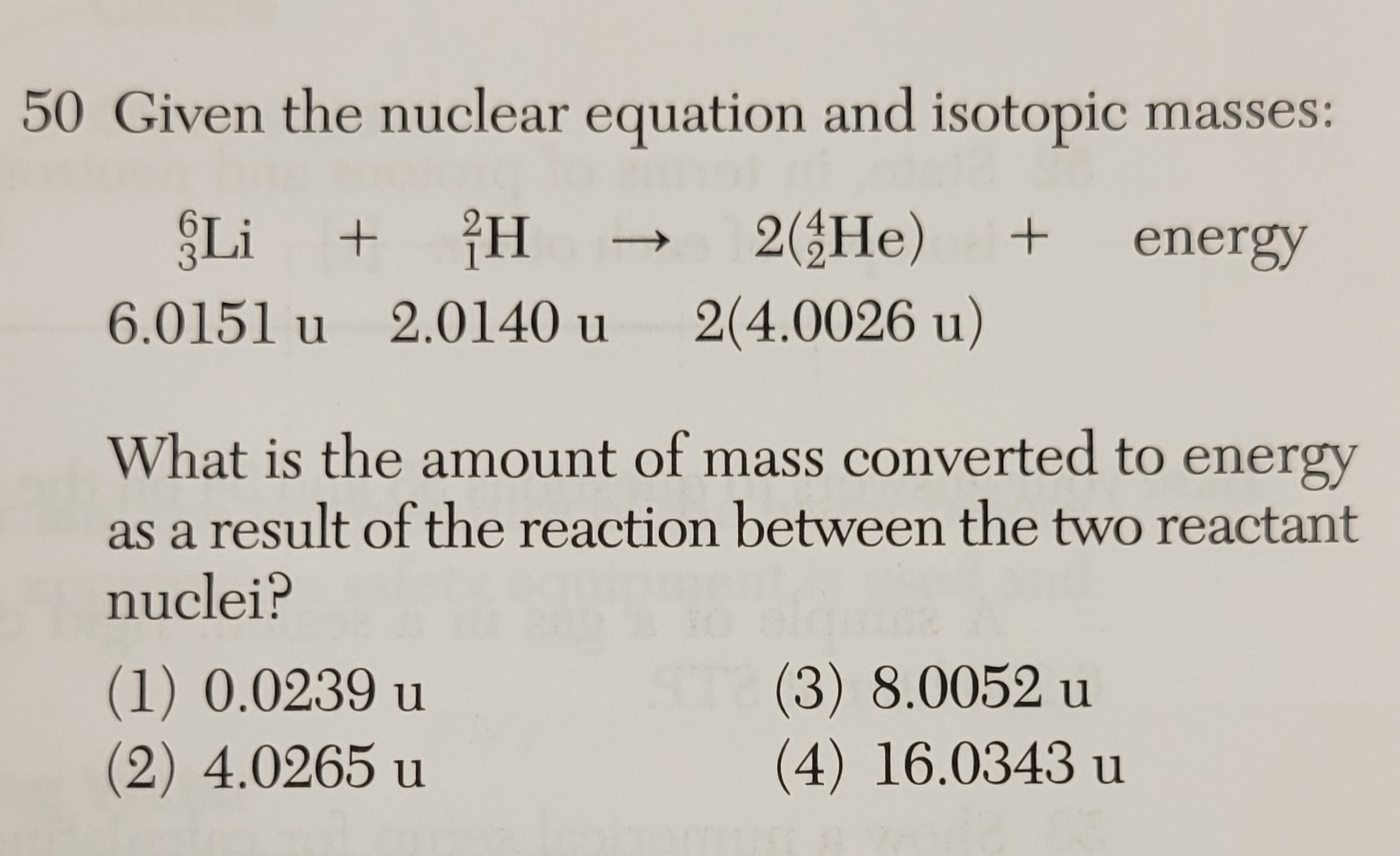
in 6.0151 +2.0140 =8.0291 u
out
2 x 4.0026 =8.0052 u
missing mass = 8.0291-8.0052=0.0239u