|
|
|
|
|
During a laboratory activity, a student
heats a beaker containing 120.0 grams of
water as shown in the diagram.
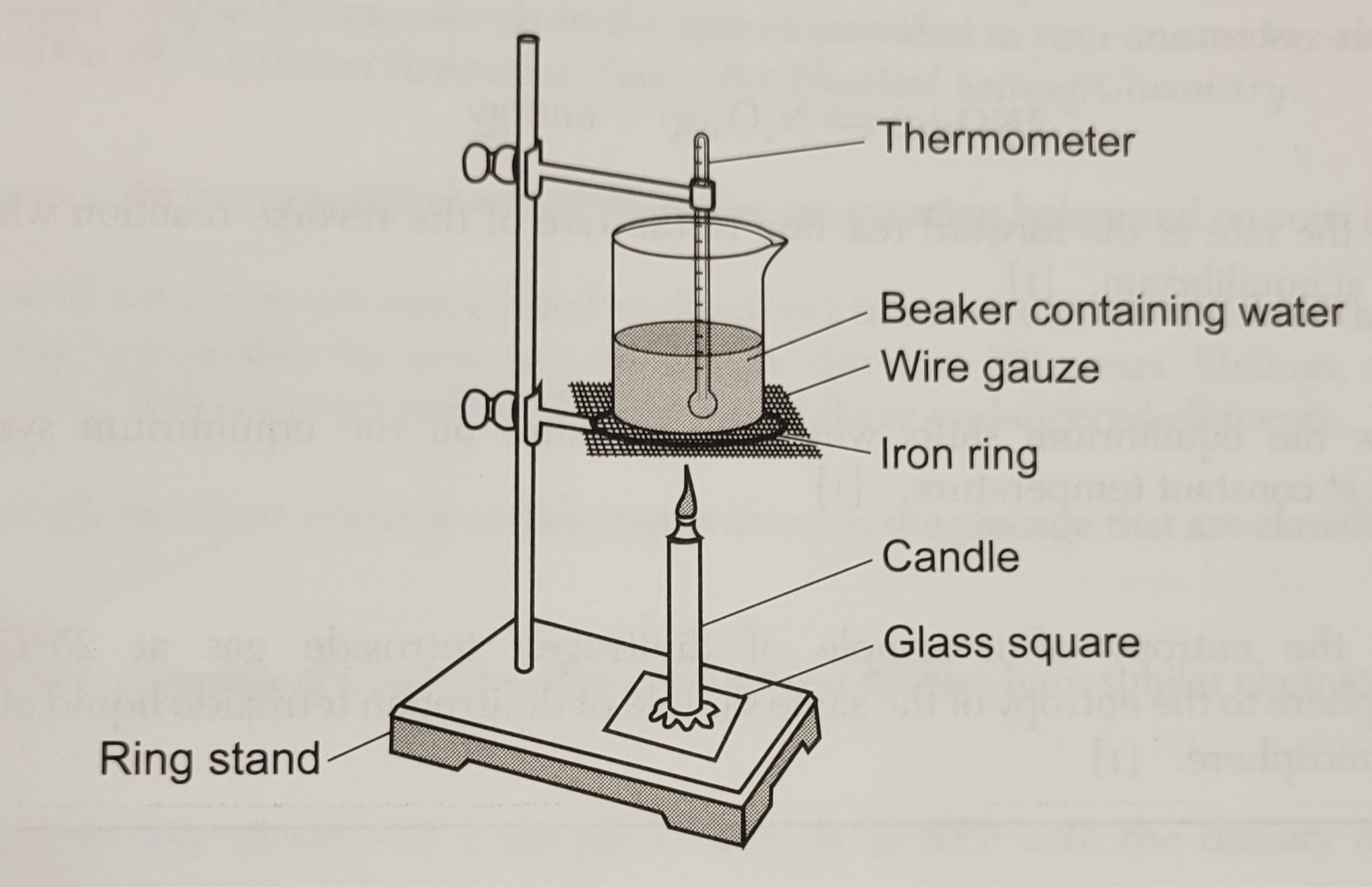
The table below shows the mass of water and the
temperature of the water before and after
heating. During this laboratory activity,
appropriate safety equipment is used and safety
procedures are followed.
|
Data for Heating Water |
| Mass of 120.0 mL of water |
120.0 g |
| Temperature of water before heating |
23.0 oC |
|
Temperature of water after heating 20.0
min |
86.0
oC |
|
55 State the
direction of heat flow between the
candle flame and the beaker of water
during the time the candle is lit.
| Answer-->
Candle
flame (hot) to the beaker of
water (cold) |
56 Show a
numerical setup for calculating
the amount of heat, in joules,
absorbed by the water in the
beaker as a result of the
burning candle.
| Answer-->
q=mCDT
q=120.0g x 4.18 J/gC x (86.0oC-23.0oC)
q=120* 4.18*63 |
57 State how the
molecular motion of the water
molecules in the beaker changes as
the temperature increases.
| Answer-->
The molecular
motion is faster, more
energetic, more entropic |
|
see
Table F halides
|
|
|
|