|
|
|
Questions
|
Answer |
Links |
Explanations
|
31. The numbers of protons, neutrons, and electrons in each of four
different ions are shown in the table below. Which ion has the
greatest mass?
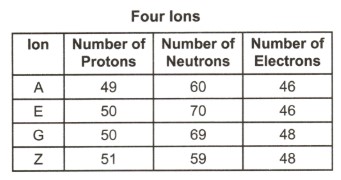
1 A
2 E
3 G
4 Z |
2 |
|
ignore electrons, they have a tiny mass protons and
neutrons are 1u
A=109
E=120
G=119
Z=110 |
32. The mass of a sample of nickel is determined to be
20.40 grams. How many significant figures are used to express this
mass?
1
2
3
4 |
4 |
|
trailing zeros count if there is a decimal |
33. What is a chemical name for the compound PbO₂?
1 Lead(I) oxide
2 Lead(II) oxide
3 Lead(III) oxide
4 Lead(IV) oxide |
4 |
|
The roman numeral is the charge of the lead Pb=4+
O each are 2- |
|
|
34. Which formula is the empirical formula for ethane, C₂H₆?
1 CH
2 CH₂
3 C₂H₃
4 CH₃ |
4 |
|
empirical formula is the reduced formula |
| 35. Given a balanced equation representing a reaction:
2CO(g) + O2(g) --> 2CO2(g) + ENERGY
Which mass of O2(g) reacts completely with 5.6 grams
of CO(g) to produce 8.8 grams of CO2(g)?
1. 1.6g
3 3.2g
2. 2.8g
4 14.4g |
3 |
|
8.8g = 5.6g + X X= 3.2g |
|
|
