|
Hydrogen gas is needed for many industrial
chemical reactions. An equilibrium system
that includes the use of a catalyst produces
hydrogen from methane and steam is shown in
equation 1 below.
Equation 1: CH4(g) + H2O(g)
+ 210 kJ <=>CO(g) + 3H2(g)
Another equilibrium system produces
hydrogen gas as represented by
equation 2 below.
Equation 2: CO(g) + HCO(g) <=>CO(g)
+ 3H2(g)+ energy
70
Compare the rate of the forward
reaction to the rate of the reverse
reaction for the equilibrium system
at equilibrium represented by
equation 1.
71 State how the
equilibrium shifts when the
temperature of the equilibrium
system at equilibrium
represented by equation 1 is
increased.
72 State, in terms of
activation energy, why the
catalyst increases the rate of
the forward reaction represented
in equation 1.
| Answer--> lowers the
activation energy |
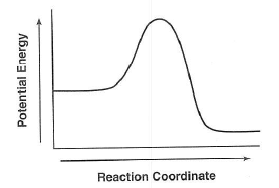
73 On the labeled
axes in your answer booklet,
draw a potential energy diagram
for the forward reaction
represented in equation 2.
| Answer--> exothermic
PE diagram
 |
|