where the formula weight (FW) is in g/mol, and the standard molar volume is 22.4 L/mol. Now try using this in a problem.
What is the density of helium gas at STP?
If the density of the gas is equal to 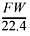 , then FW= 4.00 g/mol, 22.4 L/mol, so the density = 0.179 g/L.
, then FW= 4.00 g/mol, 22.4 L/mol, so the density = 0.179 g/L.

