|
More Gas Law links | | Mole ConversionsThe Mole Bridge | |
How to use this mole bridge: 1. You are only allowed to go from left to right. 2. The great divide side, you will divide. 3. On the Rabittville, side you will be multiplying (rabbits multiply). Those are the rules |
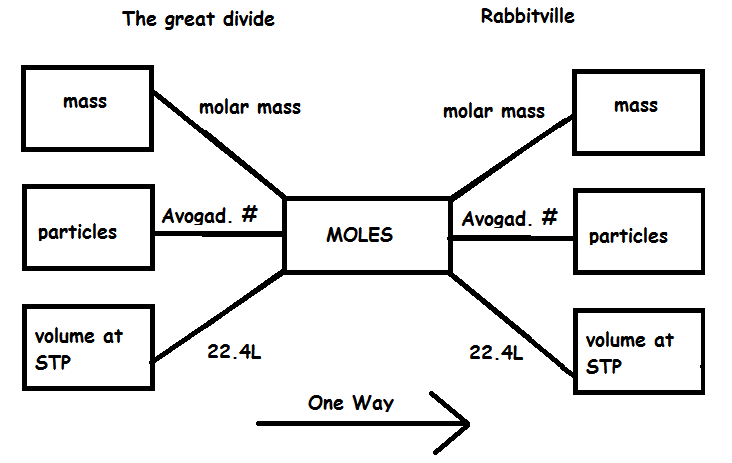
Mass to moles- divide by the molar mass 36.0g H2O | 36.0g H2O X | 1 mole | =2.00 moles water | 18.0g |
66.0g CO2 | 66.0g CO2 X | 1 mole | =1.50 moles CO2 | 44.0g |
Moles to mass- multiply by the molar mass | 5.0 Moles of He atoms | 5.0 Moles X | 4.00g | =20.0g He atoms | | 1 mole | | 30.0 moles of H2 | 30.0 moles X | 2.00g | =60.0g of H2 | | 1 mole |
Moles to particles-multiply by Avagodro's Number | 5.0 Moles of Fe atoms | 5.0 Moles X | 6.02×1023 | =3.0 x 1024 Fe atoms | | 1 mole | | 30.0 moles of H2 | 30.0 moles X | 6.02×1023 | =1.8 x 1025 H2 molecules | | 1 mole |
Particles to moles-divide by Avagodro's Number 1.8 x 1024 Donuts | 1.8 x 1024 X | 1 mole | =3.0 moles Donuts | 6.02×1023 | | 3.0 x 1025 O2 molecules | 3.0 x 1025 X | 1 mole | =50 moles O2 molecules | 6.02×1023 | | 5.4 x 1022 Molecules of Glucose | 5.4 x 1022 X | 1 mole | =0.090 moles of Glucose | 6.02×1023 |
Vol. at STP to moles-divide by 22.4L | 44.8L of Helium at STP | 44.8L He X | 1 mole | =2.00 moles of Helium | 22.4L |
Moles to Volume at STP-multiply by 22.4L | 2.5 moles of H2 | 2.5 moles X | 22.4L | = 56.0L of H2 | | 1 mole |
 Chemical Demonstration Videos Chemical Demonstration Videos
| 
