
 Custom Search back to Kinetics and Equilibrium links
Standard Enthalpy of Formation ΔHөfProperties of a substance that don't depend on its History are called State Functions. One of the most important State functions for a Chemical system is the Enthalpy, because it tells us the ability to produce Heat, a form of Energy. The problem is that we can only measure CHANGES in the Enthalpy of the system, and have no way to determine the ABSOLUTE Enthalpy. We have to define a convenient ZERO of Enthalpy for all the chemical compounds that we can make. We do this by defining the enthalpy of the Standard State of the Elements, which is only about 100 definitions and should cover all possible matter since all matter is made of elements. The Enthalpy of an Element in its Standard State at 298K is Defined as Zero. The Standard State of an element is defined as pure, at 1 atmosphere pressure, and in the phase it would normally occur at 298K. Thus, the standard state of Argon is Ar(gas), Oxygen is O2 (gas), Carbon is C(solid, graphite), and Bromine is Br2 (liquid) We can now define the Enthalpy of any molecule in any phase at 298 K through its Standard Heat of Formation, DHfo. The Heat of Formation, DHfo, is the heat evolved from the synthesis reaction of one mole of the substance from the Standard State of its constituent elements. The DHfo(Diamond) is not zero because it is not the Standard State of the element Carbon. Examples C (s) + O2 (g) ---> CO2 (g) 2 Ag (s) + 1/8 S8 (s) ---> Ag2S (s) ***Note all the products have a coefficient of 1.*** and There is never a compound on the reactant side, only elements. The superscript o means that all pressures are 1 atmosphere and all concentrations (for solutions) are 1 molar. ΔH = ∑ ΔHөf (products) - ∑ ΔHөf (reactants)
Example
Answer 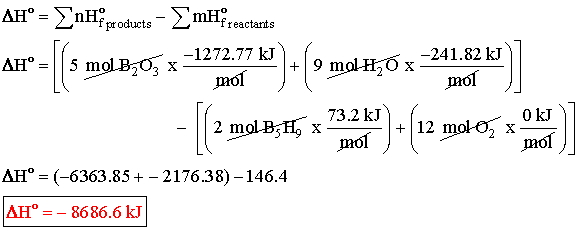 |