
Custom Search back to Kinetics and Equilibrium links
What is Ksp?
How does an Ionic Compound dissolve? The simplest way to describe this is the cation and the anions dissociate (break apart) into individual ions. Note****polyatomic ions stay together. How do we write a chemical expression of dissolving? When writing an ionic equation, any subscripts of anions and cations now become coefficients on the product side (right of the arrow). You must include the charges and the phase is now aqueous.
PbCrO4(s) <=> Pb2+(aq) + CrO42-(aq) AgCl(s) <=> Ag+(aq) + Cl-(aq) CaCO3(s) <=> Ca2+(aq) + CO32-(aq) BaSO4 (s) <-->Ba2+ (aq) + SO42- (aq)
Practice writing equations-Highlight to reveal answer
Writing the Ksp expression Used when an ionic compound dissolves into its ions. For the reaction:
Since the concentration of the solid is a constant, it is effectively incorporated into the equilibrium constant: Ksp = [B]b[C]c
Determining Ksp from Solubility ***Solubility = X*** There are 4 different versions of an ionic compound dissolving
Let's see how this plays out
Example 1 AgBr (2 ion salt ksp =x2) Determine the Ksp of silver bromide, given that its molar solubility is 5.71 x 10¯7 moles per liter. When AgBr dissolves, it dissociates like this:
The Ksp expression is: Ksp = [Ag+] [Br¯] Putting the values into the Ksp expression, we obtain: Ksp = (5.71 x 10¯7) (5.71 x 10¯7) = 3.26 x 10¯13 or Ksp= X2
Calculating solubility from Ksp
(2 ion salt ksp =x2 and X is the solubility) NORMAL Determine the molar solubility of silver bromide, given that its Ksp= 3.26 x 10¯13. When AgBr dissolves, it dissociates like this:
Putting the values into the Ksp expression, we obtain: Ksp = [Ag+] [Br¯] Ksp = (X) (X) = 3.26 x 10¯13 X2=3.26 x 10¯13 X=5.71 x 10¯7M Example 3 (3 ion salt Ksp= 4x3, X is the solubility in moles/L) HARDER Calculate the solubility of CaF2 in g/L (Ksp = 4.0 x 10-8) First, write the BALANCED REACTION: Next, set up the SOLUBILITY PRODUCT EQUILIBRIUM EXPRESSION: In the above equation, however, we have two unknowns, [Ca2+] and [F-]2. So, we have to write one in terms of the other using mole ratios. According to the balanced equation, for every one mole of Ca2+ formed, 2 moles of F- are formed. To simplify things a little, let's assign the variable X for the solubility of the Ca2+: If we SUBSTITUTE these values into the equilibrium expression, we now only have one variable to worry about, X: 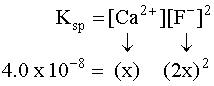 We can now SOLVE for X: 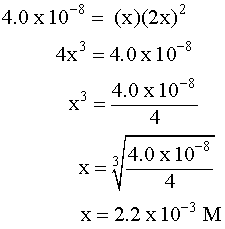 We assigned X as the solubility of the Ca2+ which is equal to the solubility of the salt, CaF2. However, our units right now are in molarity (mol/L), so we have to convert to grams: 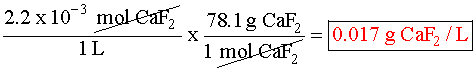
1. The solubility of PbCrO4 is 1.34 x 10-7 mol/L at 25ēC. Calculate the value of Ksp.
2. The Ksp for AgCl is 1.7 x 10-10 at 25ēC. What is the solubility of AgCl?
|