
 Custom Search link to the Periodic Trends of Electron Affinity back to Periodic Table Links First Ionization Energy Ionization Energy- the energy required to remove the most loosely held electron of an atom in the gas phase M(g)--> M(g)+ + e-
Think of ionization energy as the energy to "super excite" an electron. No just to make it jump an energy level or 2, but to actually make it leave the atom. 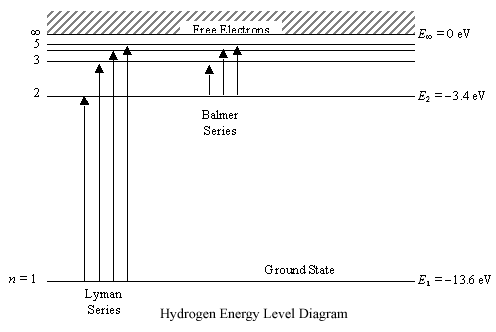 The Rubber band Analogy Place a rubber band on your thumb. Pull it. This puts energy in and the rubber band gets further from your thumb. Pull too hard and the rubber band may break free. Now you have a thumb and a separate rubber band. Back to the atom Outer electron is the rubber band and the rest of the atom is your thumb. Add too much energy and the electron is removed. This energy is ionization energy. Ionization Energy and Atoms Back to the rubber band. If the rubber band is already stretched, you will not need to add as much energy (as one that is not stretched) to remove it. Back to the Atom-The further the electron is from the nucleus, the less energy it will require to remove the electron.
(Groups) Ionization energy is related to the atomic size (radii of an atom). The smaller it is the more energy that is required to remove an electron. This works nicely for explaining the difference between elements in the same group of the Periodic Table. He has one less energy level (smaller) than Ne, so the ionization energy is greater.
(Periods) Let's look at the trend of Li to Ne (across Period 2). There are a few dips. Why?
As for the Dip from Ne to Na, Ne has a full 2 principal energy level and Sodium has a partially filled 3rd principal energy level. Neon is also much smaller.
An additional way to explain this trend is the use of the following key term. KEY TERM- Effective Nuclear Charge- the charge felt by the outer electron after distance and shielding of other electrons are taken into account. Shielding- The blocking by inner electrons of the charge of the nucleus to the outer electrons. "The more effective the nuclear charge the more energy required to remove the electron." link to the Periodic Trends Atomic Radii back to Periodic Table Links |