| Reduction-oxidation (redox) reactions are chemical reactions in which reactants experience a change in oxidation number (which means these reactants either gain or lose electrons). If an atom in a reactant gained electrons (its oxidation # decreased) it was reduced. If an atom in a reactant lost electrons (its oxidation # increased) it was oxidized. Since chemical reactions don't make or destroy electrons, oxidation and reduction must occur at the same time. As one reactant is oxidized, the electrons it loses are accepted by another reactant which is reduced. Cu2+ + Zn --> Cu + Zn2+ The Cu2+ is reduced (ox. # decreased). The Zn is oxidized (ox. # increased). Past Regents Questions involving Oxidation or Reduction June 2007-22 Which changes occur when Pt2+ is reduced?
(1) The Pt2+ gains electrons and its oxidation number increases.
(2) The Pt2+ gains electrons and its oxidation number decreases.
(3) The Pt2+ loses electrons and its oxidation number increases.
(4) The Pt2+ loses electrons and its oxidation number decreases. Aug 2006-24 Given the balanced equation representing a redox reaction:
2Al + 3Cu2+ --> 2Al3+ + 3Cu
Which statement is true about this reaction?
(1) Each Al loses 2e- and each Cu2+ gains 3e-
(2) Each Al loses 3e- and each Cu2+ gains 2e-
(3) Each Al3+ gains 2e- and each Cu loses 3e-
(4) Each Al3+ gains 3e- and each Cu loses 2e- Jan 2006-20 In an oxidation-reduction reaction, reduction is defined as the
(1) loss of protons (3) loss of electrons
(2) gain of protons (4) gain of electrons Jan 2005-23 Which change in oxidation number indicates oxidation?
(1) –1 to +2 (3) +2 to –3
(2) –1 to –2 (4) +3 to +2 Aug 2003-27. Given the reaction: 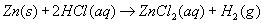
Which statement correctly describes what occurs when this reaction takes place in a closed system? A. Atoms of Zn(s) lose electrons and are oxidized.
B. Atoms of Zn(s) gain electrons and are reduced.
C. There is a net loss of mass.
D. There is a net gain of mass. Aug 2003-21. Which type of reaction occurs when nonmetal atoms become negative nonmetal ions? A. oxidation
B. reduction
C. substitution
D. condensation Jan 2003-27 When a neutral atom undergoes oxidation, the atom’s oxidation state (1) decreases as it gains electrons
(2) decreases as it loses electrons
(3) increases as it gains electrons
(4) increases as it loses electrons
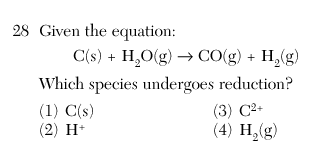
Aug 2002-22 In any redox reaction, the substance that undergoes reduction will
(1) lose electrons and have a decrease in oxidation number
(2) lose electrons and have an increase in oxidation number
(3) gain electrons and have a decrease in oxidation number
(4) gain electrons and have an increase in oxidation number June 2002- 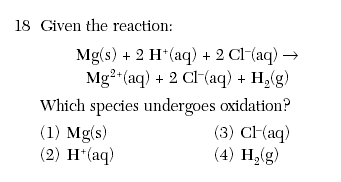
| 
