| | Properties of Ionic Compounds | |
Some Common Features of Materials with Ionic Bonds: Hard Form crystal lattices not molecules Good insulators High melting points/ Boiling Points Conduct electricity when dissolved in water or as a liquid. Solids do not conduct electricity
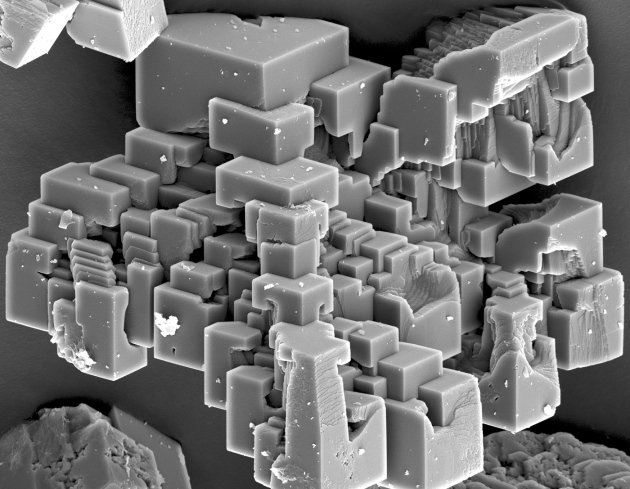 Crystal of CaCO3 | NaCl(s) | NaCl(l) | NaCl(aq) | | nonconductor | conductor | conductor |
Key Words for IONIC-Crystal, Salt, transfer of electrons -->ON TO COVALENT PROPERTIES  Chemical Demonstration Videos Chemical Demonstration Videos
| 
